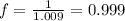Chemistry, 16.04.2020 04:57 pleasedontspamme
You prepare a buffer solution from 10.0 mL of 0.100 M MOPS (3‑morpholinopropane‑1‑sulfonic acid) and 10.0 mL of 0.074 M NaOH . 0.074 M NaOH. Next, you add 1.00 mL of 3.51 × 10 − 5 M 3.51×10−5 M lidocaine to this mixture. Denoting lidocaine as L, calculate the fraction of lidocaine present in the form LH +

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
You prepare a buffer solution from 10.0 mL of 0.100 M MOPS (3‑morpholinopropane‑1‑sulfonic acid) and...
Questions

History, 22.04.2021 19:30



Social Studies, 22.04.2021 19:30

Biology, 22.04.2021 19:30




History, 22.04.2021 19:30

Computers and Technology, 22.04.2021 19:30

Mathematics, 22.04.2021 19:30



Mathematics, 22.04.2021 19:30




English, 22.04.2021 19:30

English, 22.04.2021 19:30

Physics, 22.04.2021 19:30

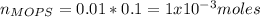
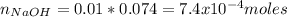
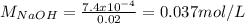
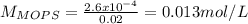
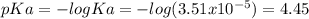
![pH=pKa+log\frac{[NaOH]}{[MOPS]} =4.45+log\frac{0.037}{0.013} =4.9](/tpl/images/0604/7273/c3441.png)
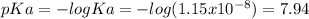
![pH=pKa+log\frac{[base]}{[acid]} \\4.9=7.94+log\frac{[base]}{[acid]}\\log\frac{[base]}{[acid]}=-3.04\\base/acid=9.12x10^{-4}](/tpl/images/0604/7273/6eb33.png)