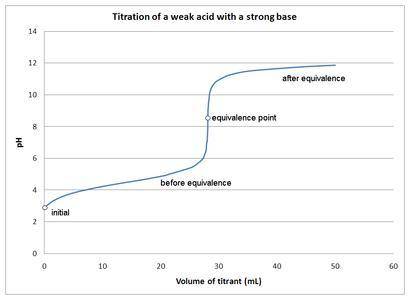
Which of the following is TRUE?
a. The equivalence point is where the amount of acid equals the amount of base during any acid-base titration.
b. At the equivalence point, the pH is always 7.
c. An indicator is not pH sensitive.
d. A titration curve is a plot of pH vs. the [base]/[acid] ratio.
e. None of the above are true.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
Which of the following is TRUE?
a. The equivalence point is where the amount of acid equ...
a. The equivalence point is where the amount of acid equ...
Questions

Mathematics, 26.07.2020 01:01





Mathematics, 26.07.2020 01:01




Mathematics, 26.07.2020 01:01



Geography, 26.07.2020 01:01


Mathematics, 26.07.2020 01:01




Computers and Technology, 26.07.2020 01:01