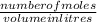Chemistry, 16.04.2020 04:15 kayranicole1
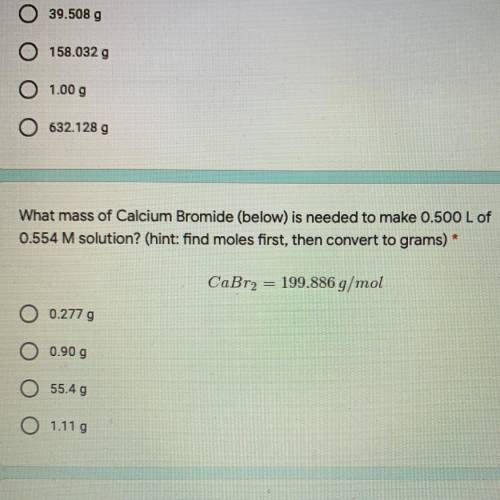
What mass of Calcium Bromide (below) is needed to make 0.500 L of
0.554 M solution? (hint: find moles first, then convert to grams)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1


Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 23.06.2019 04:31
Areaction is first order. if the initial reactant concentration is 0.0200 m, and 25.0 days later the concentration is 6.25 x 10-4 m, then its half-life is:
Answers: 1
You know the right answer?
What mass of Calcium Bromide (below) is needed to make 0.500 L of
0.554 M solution? (hint: fin...
0.554 M solution? (hint: fin...
Questions











Social Studies, 11.01.2020 02:31









 = ?
= ?