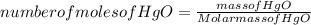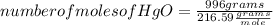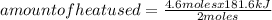Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 23.06.2019 12:30
15) a substance used in manufacturing gasoline consists of finely divided platinum supported on an inert solid. suppose that the platinum is formed by the high temperature reaction between platinum (iv) oxide and hydrogen gas. the other product is water. a) write and balance the equation b) how many grams of hydrogen are needed to produce 1.0 g of platinum metal? c) how many moles of water are produced at the same time? how many grams? ( show work, .)
Answers: 1

Chemistry, 23.06.2019 13:30
How many more valence electrons does sodium need to have a full outer valence shell
Answers: 1
You know the right answer?
A mercury mirror forms inside a test tube as a result of the thermal decomposition of mercury(ii) ox...
Questions


Mathematics, 21.04.2020 22:42




Advanced Placement (AP), 21.04.2020 22:42





History, 21.04.2020 22:42

Social Studies, 21.04.2020 22:42

Biology, 21.04.2020 22:42

Mathematics, 21.04.2020 22:42



Mathematics, 21.04.2020 22:42

Mathematics, 21.04.2020 22:42