Chemistry, 16.04.2020 02:07 EmmaPreston43
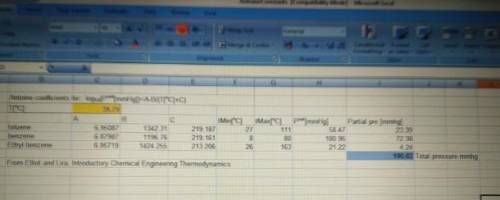
The production of ethylbenzene, a very popular industrial chemical, is carried out reacting benzene with ethylene in liquid phase. This reaction takes place in a series of reactors that involve multiple side reactions and intermediates. Ethylene, being the limiting reactant, is used up first and hence a considerable amount of benzene remains unreacted. From one of the reactors in the series, the exit stream is a mixture of this unreacted benzene (1), an intermediate – toluene (2), and the product ethyl benzene (3).
It is desirable to separate this liquid mixture before sending the components to the next series of reactors/process steps. So 100 mol/min of this mixture is flashed from 200 mm Hg and 50 °C to 100 mm Hg. If the mole fraction of benzene and toluene are 40% each when the mixture enters the flash distillation unit, determine if the mixture will flash completely, partially, or not at all. Assume ideal gas and ideal solution behavior for the vapor phase and liquid phase, respectively. If the mixture does flash partially, determine the composition and molar flow rates of the equilibrium streams exiting the reactor. Show all calculations by hand using your preferred method for solving simultaneous equations. Alternatively, you may use Solver (Excel) but this must be accompanied by a printout of a neatly formatted Excel sheet showing your equations and constraints.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Drag each number to the correct location on the equation. each number can be used more than once, but not all numbers will be used. balance the equation with the coefficients. 2 3 4 5 kclo3 -> kcl + o2
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 04:20
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1
You know the right answer?
The production of ethylbenzene, a very popular industrial chemical, is carried out reacting benzene...
Questions

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Geography, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01

Mathematics, 16.09.2020 14:01