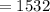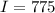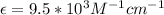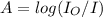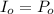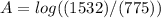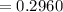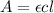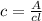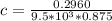Chemistry, 16.04.2020 01:01 Scienceissofun6453
Calculate the concentration of an anthracene solution which produced a fluorescence intensity ( I ) of 775 when the irradiance of the beam incident to the sample ( P 0 ) was 1532 and the length of the medium ( b ) was 0.875 cm. Anthracene has a molar extinction coefficient ( ϵ ) of 9.5 × 10 3 M − 1 ⋅ cm − 1 . The proportionality constant k ′ for anthracene is 0.30.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

Chemistry, 23.06.2019 06:20
Type the correct answer in each box.balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1
You know the right answer?
Calculate the concentration of an anthracene solution which produced a fluorescence intensity ( I )...
Questions

English, 28.12.2020 15:50


Mathematics, 28.12.2020 15:50


English, 28.12.2020 15:50


English, 28.12.2020 15:50







English, 28.12.2020 16:00




Mathematics, 28.12.2020 16:00


History, 28.12.2020 16:00

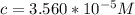
 is
is