
The following reaction was used to fuel the rockets in the Apollo mission landing module.
A) Is this reaction endothermic or exothermic?
B) How many grams of N2H4 must be reacted with an excess of N2O4 to produce 775 kJ of energy?
C) How many kJ of energy are produced when 6.25 g of N2O4 reacts with an excess of N2H4?
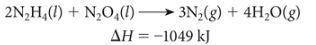

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2
You know the right answer?
The following reaction was used to fuel the rockets in the Apollo mission landing module.
Questions


Social Studies, 30.08.2019 18:00

History, 30.08.2019 18:00


English, 30.08.2019 18:00


Physics, 30.08.2019 18:00





History, 30.08.2019 18:00

Mathematics, 30.08.2019 18:00



Biology, 30.08.2019 18:00




Mathematics, 30.08.2019 18:00



