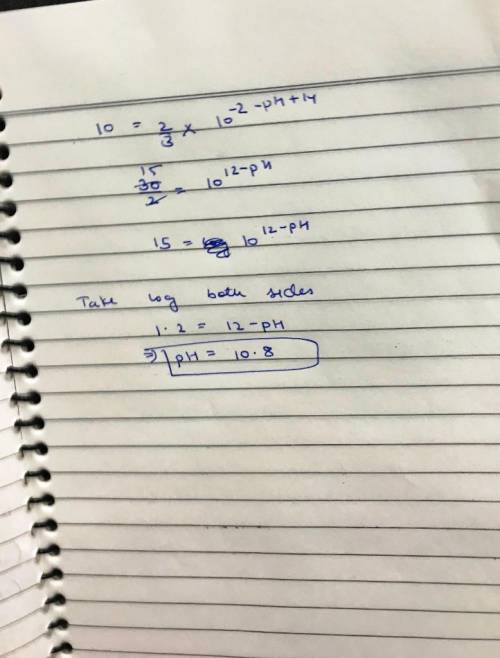Chemistry, 16.04.2020 00:17 sarahhN7534
The decomposition of Bromodichloroacetate BrCl2CCO2- is an important required step in water purification. The kinetics of such decomposition has been presented in Chemical Reviews, November 2001. There are two possible pathways for these reactions, one unimolecular and the other bimolecular with the help of OH- ions.
Path 1. BrCl2CCO2- + H2O goes to CHCl2Br + HCO3-
With a pseudo-first-order rate constant k1=1.6 x 10-6 1/sec
Path 2. BrCl2CCO2- + OH- goes to Cl2OHCCO2- + Br-
With a second-order rate constant k2=2.4x10-4 1/(M sec)
(a) Write the overall rate law for the disappearance of BrCl2CCO2.
(b) What is the ratio of the rates for paths 1 and 2 at pH=7?
(c) At what pH would the rate for path 2 be 10% of the rate for path 1?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
You know the right answer?
The decomposition of Bromodichloroacetate BrCl2CCO2- is an important required step in water purifica...
Questions



Physics, 02.06.2021 02:00

Chemistry, 02.06.2021 02:00

Chemistry, 02.06.2021 02:10




Mathematics, 02.06.2021 02:10

Mathematics, 02.06.2021 02:10

Mathematics, 02.06.2021 02:10



Mathematics, 02.06.2021 02:10

Mathematics, 02.06.2021 02:10

Mathematics, 02.06.2021 02:10



English, 02.06.2021 02:10

Mathematics, 02.06.2021 02:10