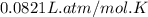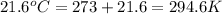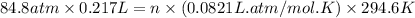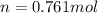Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2
You know the right answer?
A steel reaction vessel of a bomb calorimeter has a volume of 0.217 L, is charged with oxygen gas to...
Questions



English, 29.09.2019 03:30

Mathematics, 29.09.2019 03:30

Mathematics, 29.09.2019 03:30


Biology, 29.09.2019 03:30

English, 29.09.2019 03:30



Mathematics, 29.09.2019 03:30

Arts, 29.09.2019 03:30


Chemistry, 29.09.2019 03:30



Mathematics, 29.09.2019 03:30

Mathematics, 29.09.2019 03:30

History, 29.09.2019 03:30

History, 29.09.2019 03:30

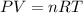
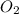 gas = 84.8 atm
gas = 84.8 atm