

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2
You know the right answer?
AB2 is a molecule that reacts readily with water. Calculate the bond energy of the A–B bond using th...
Questions

Chemistry, 25.03.2020 07:49


Geography, 25.03.2020 07:50

Mathematics, 25.03.2020 07:50

English, 25.03.2020 07:50


Mathematics, 25.03.2020 07:51

Mathematics, 25.03.2020 07:51

History, 25.03.2020 07:51


Chemistry, 25.03.2020 07:51


History, 25.03.2020 07:51

Mathematics, 25.03.2020 07:51

Chemistry, 25.03.2020 07:51


English, 25.03.2020 07:51



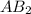 is a molecule that reacts readily with water. Calculate the bond energy of the A–B bond using the standard enthalpy of reaction and the bond energy data provided. Enter a number in kJ to 0 decimal places.
is a molecule that reacts readily with water. Calculate the bond energy of the A–B bond using the standard enthalpy of reaction and the bond energy data provided. Enter a number in kJ to 0 decimal places. 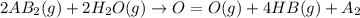
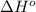 = –142 kJ
= –142 kJ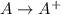
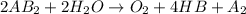
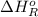 = -142 kJ
= -142 kJ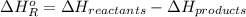
![[(2 \times 2 (A-B) + 2 \times 2 (O-H)] - [(O=O) + 4(H-B) + (A-A)]](/tpl/images/0603/6629/cfc5d.png)
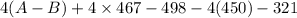
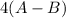 = -142 - 1868 + 498 + 1800 + 321
= -142 - 1868 + 498 + 1800 + 321

