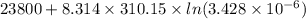The standard free-energy change for this reaction in the direction written is 23.8 kJ/mol. The concentrationsof the three intermediates in the hepatocyte of a mammal are: fructose 1,6-bisphosphate,1.4X10-5 M; glyceraldehyde 3-phosphate, 3X10-6 M; and dihydroxyacetone phosphate, 1.6X10-5 M. At body temperature (37C), what is the actual free-energy change for the reaction

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
The standard free-energy change for this reaction in the direction written is 23.8 kJ/mol. The conce...
Questions


Mathematics, 07.01.2021 01:40

Mathematics, 07.01.2021 01:40


English, 07.01.2021 01:40



Mathematics, 07.01.2021 01:40




Mathematics, 07.01.2021 01:40

Mathematics, 07.01.2021 01:40

Health, 07.01.2021 01:40

Mathematics, 07.01.2021 01:40

Mathematics, 07.01.2021 01:40




History, 07.01.2021 01:40

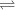 Glyceraldehyde 3-phosphate + DHAP
Glyceraldehyde 3-phosphate + DHAP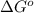 is 23.8 kJ/mol.
is 23.8 kJ/mol. 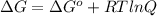
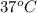
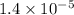 M
M
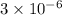 M
M
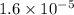 M
M ![\frac{[DHAP][\text{glyceraldehyde 3-phosphate}]}{[/text{Fructose 1,6-bisphosphate}]}](/tpl/images/0603/3958/6ad8b.png)
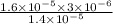
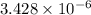
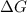 as follows.
as follows.