
You have a solution of two volatile liquids, A and B (assume ideal behavior). Pure liquid A has a vapor pressure of 385.0 torr and pure liquid B has a vapor pressure of 104.0 torr at the temperature of the solution. The vapor at equilibrium above the solution has double the mole fraction of substance A as the solution does. What is the mole fraction of liquid A in the solution?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3


Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3
You know the right answer?
You have a solution of two volatile liquids, A and B (assume ideal behavior). Pure liquid A has a va...
Questions



Mathematics, 04.11.2020 01:30

History, 04.11.2020 01:30

Mathematics, 04.11.2020 01:30




Advanced Placement (AP), 04.11.2020 01:30

Mathematics, 04.11.2020 01:30

English, 04.11.2020 01:30



History, 04.11.2020 01:30






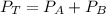 ---------- Equation (1)
---------- Equation (1)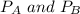 are partial pressure of A and B respectively.
are partial pressure of A and B respectively.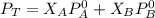 ---------- Equation (2)
---------- Equation (2)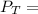 the total vapor pressure of the solution
the total vapor pressure of the solution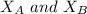 = mole fraction of A and B respectively
= mole fraction of A and B respectively 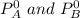 = vapor pressures of pure species of A and B
= vapor pressures of pure species of A and B 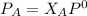
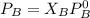
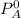 and
and 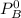 into equation (2) ; we have:
into equation (2) ; we have: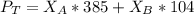
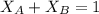
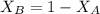
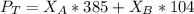
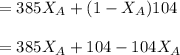
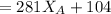 ------ Equation (3)
------ Equation (3)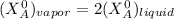
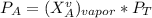
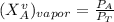
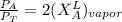 ------- Equation (4)
------- Equation (4)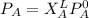
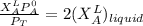
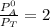
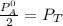
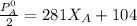
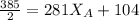
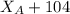
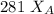
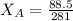
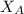 = 0.32
= 0.32 


