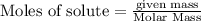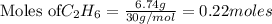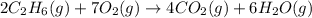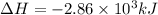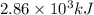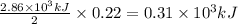Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 23.06.2019 03:00
Analyze the reaction to determine whether the reaction is exothermic or endothermic. explain your reasoning.
Answers: 1
You know the right answer?
The following thermochemical equation is for the reaction of ethane(g) with oxygen(g) to form carbon...
Questions


Advanced Placement (AP), 29.11.2020 23:20


Mathematics, 29.11.2020 23:20




Geography, 29.11.2020 23:20

Mathematics, 29.11.2020 23:20


Mathematics, 29.11.2020 23:20


Mathematics, 29.11.2020 23:20


Mathematics, 29.11.2020 23:20

Mathematics, 29.11.2020 23:20


Mathematics, 29.11.2020 23:20

English, 29.11.2020 23:20

Biology, 29.11.2020 23:20

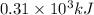 of energy is produced
of energy is produced