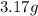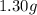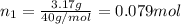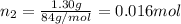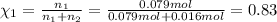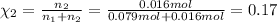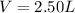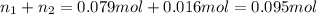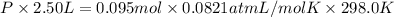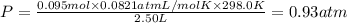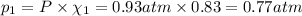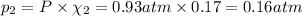Chemistry, 15.04.2020 22:05 cjjjjjjjjjjjjj
If a gaseous mixture is made by combining 3.17 g Ar 3.17 g Ar and 1.30 g Kr 1.30 g Kr in an evacuated 2.50 L container at 25.0 ∘ C, 25.0 ∘C, what are the partial pressures of each gas, P Ar PAr and P Kr , PKr, and what is the total pressure, P total , Ptotal, exerted by the gaseous mixture?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the study of how matter and energy interact? a. biology b. physics c. planetary science d. chemistry
Answers: 1

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
If a gaseous mixture is made by combining 3.17 g Ar 3.17 g Ar and 1.30 g Kr 1.30 g Kr in an evacuate...
Questions



Health, 21.09.2019 04:30

Health, 21.09.2019 04:30




Computers and Technology, 21.09.2019 04:30




Mathematics, 21.09.2019 04:30


Geography, 21.09.2019 04:30

Health, 21.09.2019 04:30

English, 21.09.2019 04:30


Social Studies, 21.09.2019 04:30

History, 21.09.2019 04:30

Computers and Technology, 21.09.2019 04:30