Chemistry, 15.04.2020 21:59 chloejason8375
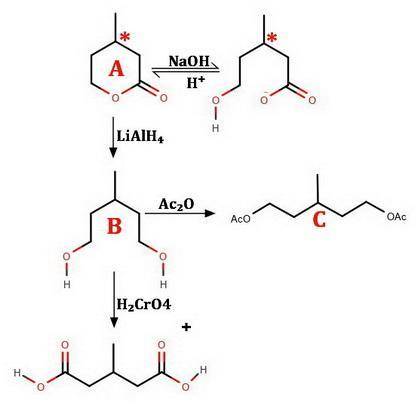
An optically active compound A, C6H10O2, when dissolved in NaOH solution, consumed one equivalent of base. On acidification, compound A was slowly regenerated. Treatment of A with LiAlH4 in ether followed by protonolysis gave an optically inactive compound B that reacted with acetic anhydride to give an acetate diester derivative C. Compound B was oxidized by aqueous chromic acid to β-methylglutaric acid (3-methylpentanedioic acid), D. Identify compounds A, B, and C; do not specify stereochemistry. (The absolute stereochemical configurations of chiral substances cannot be determined from the data.)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Which of the following might a chemist choose to study? a. glacier movement in alaska b. better ways to recycle plastics c. the effects of hurricanes on turtle populations d. the vibrations in bridges caused by big trucks
Answers: 2

Chemistry, 21.06.2019 18:00
Now consider the reaction when 45.0 g naoh have been added. what amount of naoh is this, and what amount of fecl3 can be consumed by it?
Answers: 3

Chemistry, 21.06.2019 19:30
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

You know the right answer?
An optically active compound A, C6H10O2, when dissolved in NaOH solution, consumed one equivalent of...
Questions

Mathematics, 30.04.2021 18:30


Mathematics, 30.04.2021 18:30

Mathematics, 30.04.2021 18:30


Mathematics, 30.04.2021 18:30




History, 30.04.2021 18:30

History, 30.04.2021 18:30

Mathematics, 30.04.2021 18:30


English, 30.04.2021 18:30

Mathematics, 30.04.2021 18:30

Mathematics, 30.04.2021 18:30


Mathematics, 30.04.2021 18:30


Mathematics, 30.04.2021 18:30