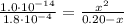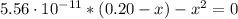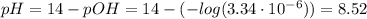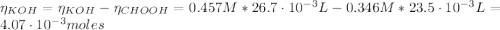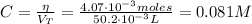Chemistry, 16.04.2020 00:36 RoxanneDuartee
Determine the pH during the titration of 23.5 mL of 0.346 M formic acid (Ka = 1.8×10-4) by 0.457 M KOH at the following points.
(a) Before the addition of any KOH
(b) After the addition of 4.00 mL of KOH
(c) At the half-equivalence point (the titration midpoint)
(d) At the equivalence point
(e) After the addition of 26.7 mL of KOH

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
You know the right answer?
Determine the pH during the titration of 23.5 mL of 0.346 M formic acid (Ka = 1.8×10-4) by 0.457 M K...
Questions


English, 31.08.2019 08:00

Biology, 31.08.2019 08:00

Social Studies, 31.08.2019 08:00

History, 31.08.2019 08:00


History, 31.08.2019 08:00


History, 31.08.2019 08:00



Biology, 31.08.2019 08:00

Biology, 31.08.2019 08:00


Mathematics, 31.08.2019 08:00


Health, 31.08.2019 08:00


History, 31.08.2019 08:00

![pH = -log([H_{3}O^{+}])](/tpl/images/0603/8843/6ab72.png) (1)
(1) ![K_{a} = \frac{[CHOO^{-}][H_{3}O^{+}]}{[CHOOH]}](/tpl/images/0603/8843/c4333.png) (3)
(3)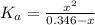
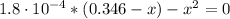 (4)
(4)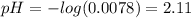
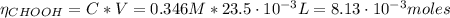
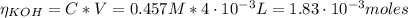
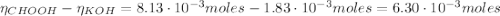
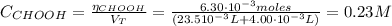
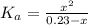
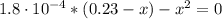
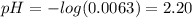
![pH = pKa + log(\frac{[CHOO^{-}]}{[CHOOH]})](/tpl/images/0603/8843/112dd.png)
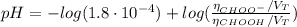
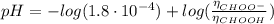
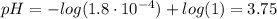
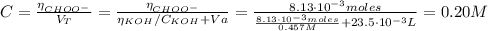
![K_{b} = \frac{[CHOOH][OH^{-}]}{[CHOO^{-}]}](/tpl/images/0603/8843/a45f9.png)
![\frac{K_{w}}{K_{a}} = \frac{[CHOOH][OH^{-}]}{[CHOO^{-}]}](/tpl/images/0603/8843/7611b.png)