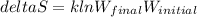Chemistry, 15.04.2020 21:08 rileyeddins1010
A gaseous system undergoes a change in temperature and volume. What is the entropy change for a particle in this system if the final number of microstates is 0.418 times that of the initial number of microstates? Express your answer numerically in joules per kelvin per particle.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2


Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
A gaseous system undergoes a change in temperature and volume. What is the entropy change for a part...
Questions





English, 29.09.2021 15:50



English, 29.09.2021 15:50


Mathematics, 29.09.2021 15:50



Mathematics, 29.09.2021 15:50

Computers and Technology, 29.09.2021 15:50




Mathematics, 29.09.2021 15:50

Physics, 29.09.2021 15:50

English, 29.09.2021 15:50