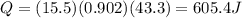Chemistry, 15.04.2020 20:56 harodkdc7910
Suppose you store a 15.5 g piece of aluminum (Cp of Al = 0.902 J/g⁰C) in the refrigerator at 2.3⁰C and then drop it into your coffee. The coffee temperature drops from 95.6⁰C to 45.6⁰C. How many Joules of energy did the aluminum block absorb?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 23.06.2019 06:30
Achemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 no(g) + cl2(g) < => 2 nocl(g) kp = 2 x 10^(-6)he fills a reaction vessel at this temperature with 13. atm of nitrogen monoxide gas and 12. atm of chlorine gas. use this data to answer the questions: a. can you predict the equilibrium pressure of noci, using only the tools available to you within aleks? y/nb. if you said yes, then enter the equilibrium pressure of nocl at right. round your answer to 1 significant digit.
Answers: 1
You know the right answer?
Suppose you store a 15.5 g piece of aluminum (Cp of Al = 0.902 J/g⁰C) in the refrigerator at 2.3⁰C a...
Questions

Chemistry, 13.11.2020 19:40

Mathematics, 13.11.2020 19:40

Mathematics, 13.11.2020 19:40

English, 13.11.2020 19:40

Mathematics, 13.11.2020 19:40

Mathematics, 13.11.2020 19:40

History, 13.11.2020 19:40


Chemistry, 13.11.2020 19:40




Mathematics, 13.11.2020 19:40


Mathematics, 13.11.2020 19:40

Chemistry, 13.11.2020 19:40

Chemistry, 13.11.2020 19:40



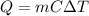
 is the change in temperature
is the change in temperature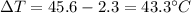 is the change in temperature of the aluminium (in fact, at thermal equilibrium, the block of aluminium reaches the same final temperature as the coffee)
is the change in temperature of the aluminium (in fact, at thermal equilibrium, the block of aluminium reaches the same final temperature as the coffee)