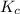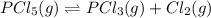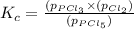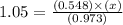Chemistry, 15.04.2020 21:03 soleydyperez
The equilibrium constant (Kp) for the decomposition of phosphorus pentachloride (PCl5) to phosphorus trichloride (PCl3) and molecular chlorine (Cl2) is found to be 1.05 at 250oC. If the equilibrium partial pressure of PCl5 and PCl3 are 0.973 and 0.548 atm, respectively, what is the equilibrium partial pressure of Cl2 at 250 oC?PCl5 (g) ↔ PCl3 (g) + Cl2 (g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
You know the right answer?
The equilibrium constant (Kp) for the decomposition of phosphorus pentachloride (PCl5) to phosphorus...
Questions

English, 25.12.2021 21:40




Mathematics, 25.12.2021 21:50



Social Studies, 25.12.2021 21:50

World Languages, 25.12.2021 21:50

Social Studies, 25.12.2021 21:50


SAT, 25.12.2021 21:50


Mathematics, 25.12.2021 21:50

Mathematics, 25.12.2021 21:50

Business, 25.12.2021 21:50


SAT, 25.12.2021 21:50

Physics, 25.12.2021 21:50

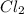 is 1.86 atm
is 1.86 atm