
Chemistry, 15.04.2020 18:26 FailingstudentXD
The reaction of Fe3O4(s) with hydrogen(g) to form iron(s) and water(g) proceeds as follows: Fe3O4(s) + 4 H2(g) 3 Fe(s) + 4 H2O(g) When 61.8 grams of Fe3O4(s) react with sufficient H2(g) , 40.3 kJ of energy are absorbed . What is the value of H for the chemical equation given?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What type of reaction is represented by the following example? 2co2 (g) + 4h2o (l) + 1452 kj 2ch3oh (l) (g) + 3o2 (g) exothermic endothermic
Answers: 1



Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1
You know the right answer?
The reaction of Fe3O4(s) with hydrogen(g) to form iron(s) and water(g) proceeds as follows: Fe3O4(s)...
Questions

Mathematics, 05.05.2020 04:06


Mathematics, 05.05.2020 04:06

Mathematics, 05.05.2020 04:06

Mathematics, 05.05.2020 04:06

Biology, 05.05.2020 04:06


Social Studies, 05.05.2020 04:06












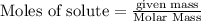
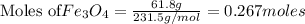
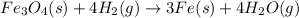
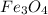 absorb energy = 40.3 kJ
absorb energy = 40.3 kJ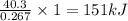
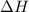 for the chemical equation given is 151 kJ
for the chemical equation given is 151 kJ

