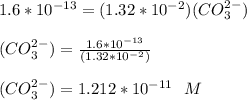A solution contains 1.32×10-2 M lead nitrate and 1.16×10-2 M copper(II) acetate. Solid sodium carbonate is added slowly to this mixture.
A. What is the formula of the substance that precipitates first? formula =
B. What is the concentration of carbonate ion when this precipitation first begins? [CO32-] = M

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1


Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2
You know the right answer?
A solution contains 1.32×10-2 M lead nitrate and 1.16×10-2 M copper(II) acetate. Solid sodium carbon...
Questions



Mathematics, 21.06.2019 23:00

Mathematics, 21.06.2019 23:00

English, 21.06.2019 23:00




Mathematics, 21.06.2019 23:00


Mathematics, 21.06.2019 23:00






English, 21.06.2019 23:00


Computers and Technology, 21.06.2019 23:00

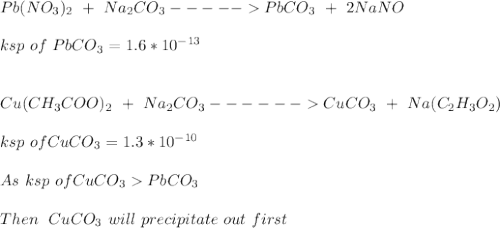
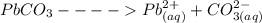
![[Pb^{2+}]](/tpl/images/0602/0905/0acfd.png) will be
will be ![[Pb(NO_3)_2] = 1.32 *10^{-2} \ M](/tpl/images/0602/0905/05cf0.png)
![ksp = [Pb^{2+}][CO^{2-}_3]](/tpl/images/0602/0905/39330.png)