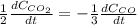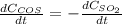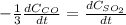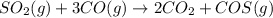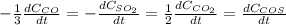Chemistry, 15.04.2020 17:39 tamariarodrigiez
Sulfur dioxide emissions in power plant stack gases may react with carbon monoxide as follows: SO_2 (g) + 3CO(g) rightarrow 2CO_2 + COS(g) Write an equation relating each of the following pairs of rates:
a. The rate of formation of CO_2 to the rate of consumption of CO
b. The rate of formation of COS to the rate of consumption of SO_2
c. The rate of consumption of CO to the rate of consumption of SO_2

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 13:30
Why hydrochloric acid neutralized first when you titrate a mixture of hcl& ch3cooh against standard sodium hydroxide
Answers: 1

Chemistry, 23.06.2019 15:40
The table below shows the freezing points of four substances. substance freezing point (°c) benzene 5.5 water 0 butane –138 nitrogen –210 the substances are placed in separate containers at room temperature, and each container is gradually cooled. which of these substances will solidify before the temperature reaches 0°c? benzene water butane nitrogen
Answers: 2

Chemistry, 23.06.2019 19:30
Atax that increases in proportion to increase in income is known as
Answers: 1

Chemistry, 24.06.2019 01:00
Ethers react with hi to form two cleavage products. one of the products might react further with hi. in the first box below draw the two major products that could be recovered after treatment with one equivalent of hi. in the second box draw the two major products that could be recovered after treatment with excess hi
Answers: 3
You know the right answer?
Sulfur dioxide emissions in power plant stack gases may react with carbon monoxide as follows: SO_2...
Questions



Mathematics, 09.01.2020 23:31

Computers and Technology, 09.01.2020 23:31

Mathematics, 09.01.2020 23:31

Physics, 09.01.2020 23:31

Mathematics, 09.01.2020 23:31


History, 09.01.2020 23:31

Mathematics, 09.01.2020 23:31

Mathematics, 09.01.2020 23:31

Biology, 09.01.2020 23:31


History, 09.01.2020 23:31

Mathematics, 09.01.2020 23:31

Mathematics, 09.01.2020 23:31


Mathematics, 09.01.2020 23:31

Biology, 09.01.2020 23:31