
Chemistry, 15.04.2020 17:31 cheyennemitchel2680
You will often work with salts of , , and in the laboratory. (All are found in nature, and all are important economically.) If you have a solution containing these three ions, each at a concentration of 0.10 M, what is the order in which their hydroxides begin to precipitate as aqueous NaOH is slowly added to the solution? Formula Enter an integer to indicate order: 1 = first, 2 = second, 3 = last : : :

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Figure 10-1 study figure 10-1. the strong nuclear force felt by a single proton in a large nucleus
Answers: 3

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1
You know the right answer?
You will often work with salts of , , and in the laboratory. (All are found in nature, and all are i...
Questions

Mathematics, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01

Physics, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01


Mathematics, 28.09.2020 14:01


Mathematics, 28.09.2020 14:01

Computers and Technology, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01


Mathematics, 28.09.2020 14:01


History, 28.09.2020 14:01

Computers and Technology, 28.09.2020 14:01



Mathematics, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01

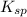 values of each individual precipitate. The lower the
values of each individual precipitate. The lower the 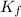 = 1.1 x 10³³)
= 1.1 x 10³³)

