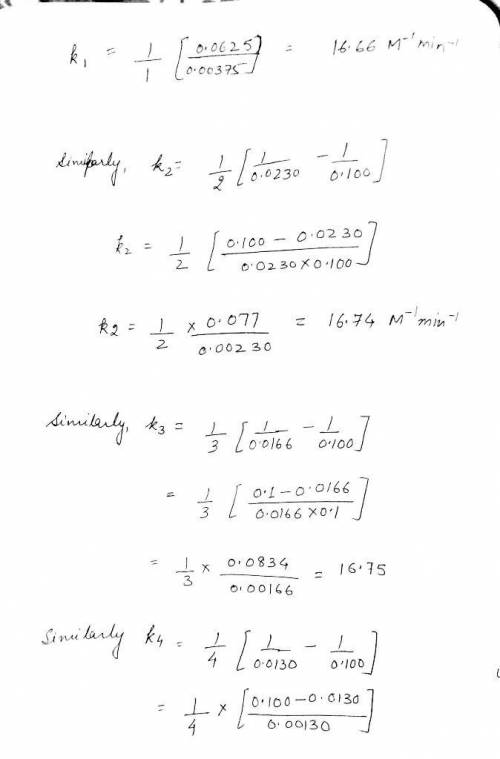2NH3g+N2g3H2g

Chemistry, 15.04.2020 16:09 aleexandras09
A chemistry graduate student is studying the rate of this reaction:
2NH3g+N2g3H2g
He fills a reaction vessel with NH3 and measures its concentration as the reaction proceeds:
time (minutes) NH3 0 0.100M 1.0 0.0375M 2.0 0.0230M 3.0 0.0166M 4.0 0.0130M
Use this data to answer the following questions.
Write the rate law for this reaction. rate =k NH3
Calculate the value of the rate constant k . Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. =k ×10

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3
You know the right answer?
A chemistry graduate student is studying the rate of this reaction:
2NH3g+N2g3H2g
2NH3g+N2g3H2g
Questions



Health, 17.07.2021 08:50

World Languages, 17.07.2021 08:50





Mathematics, 17.07.2021 08:50

German, 17.07.2021 09:10

Physics, 17.07.2021 09:10

Mathematics, 17.07.2021 09:10

Mathematics, 17.07.2021 09:10


Mathematics, 17.07.2021 09:10


World Languages, 17.07.2021 09:10

Mathematics, 17.07.2021 09:10

Social Studies, 17.07.2021 09:10

Physics, 17.07.2021 09:10