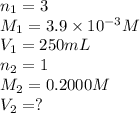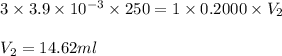Chemistry, 15.04.2020 15:52 crystalclear99
A chemistry student weighs out 0.09666 g of phosphoric acid (H3PO4), a triprotic acid, into a 250.volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.2000 M NaoH solution. Calculate the volume of NaoH solution the student will need to add to reach the final equivalence point. Round your answer to 3 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3
You know the right answer?
A chemistry student weighs out 0.09666 g of phosphoric acid (H3PO4), a triprotic acid, into a 250.vo...
Questions




Mathematics, 21.11.2020 04:20

Mathematics, 21.11.2020 04:20

Chemistry, 21.11.2020 04:20





Mathematics, 21.11.2020 04:20

Mathematics, 21.11.2020 04:20

Mathematics, 21.11.2020 04:20


Mathematics, 21.11.2020 04:20


History, 21.11.2020 04:20



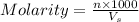
 = volume of solution in ml = 250 ml
= volume of solution in ml = 250 ml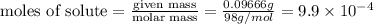
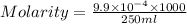
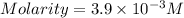
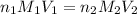
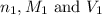 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 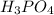
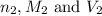 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.