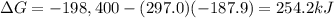For the process 2SO2(g) + O2(g) --> 2SO3(g),
ΔS = –187.9 J/K and ΔH = –198.4 kJ at 29...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

You know the right answer?
Questions

Physics, 29.01.2021 05:30


Mathematics, 29.01.2021 05:30


Biology, 29.01.2021 05:30



Mathematics, 29.01.2021 05:30

Health, 29.01.2021 05:30


Mathematics, 29.01.2021 05:30


Chemistry, 29.01.2021 05:30


Mathematics, 29.01.2021 05:30


Mathematics, 29.01.2021 05:30

History, 29.01.2021 05:30


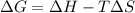
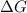 is the Gibbs free energy
is the Gibbs free energy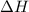 is the change in enthalpy of the reaction
is the change in enthalpy of the reaction is the change in entropy
is the change in entropy