
Chemistry, 15.04.2020 04:31 hdhdhd49jdhd
If u had 100mL of 0.1M Na2CO3 how many ML of 1M MgSO4 would you need to add in order to use up both reactants without either being left over
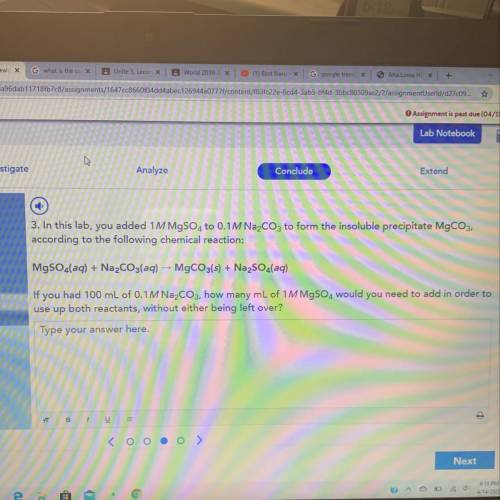

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
If u had 100mL of 0.1M Na2CO3 how many ML of 1M MgSO4 would you need to add in order to use up both...
Questions

Mathematics, 22.07.2019 00:30

Biology, 22.07.2019 00:30

Mathematics, 22.07.2019 00:30

Biology, 22.07.2019 00:30



Mathematics, 22.07.2019 00:30


Biology, 22.07.2019 00:30






English, 22.07.2019 00:30







