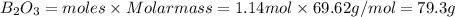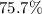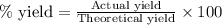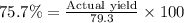Chemistry, 15.04.2020 03:45 danieldfuenteg732
If 36.9 mL of B2H6 reacted with excess oxygen gas, determine the actual yield of B2O3 if the percent yield of B2O3 was 75.7%. (The density of B2H6 is 1.131 g/mL. The molar mass of B2H6 is 27.668 g/mol and the molar mass of B2O3 is 69.62 g/mol.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which of the following is a compound? a.carbon b.oxygen c.hydrogen d.water
Answers: 2


Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3
You know the right answer?
If 36.9 mL of B2H6 reacted with excess oxygen gas, determine the actual yield of B2O3 if the percent...
Questions


Mathematics, 12.01.2022 14:00



Mathematics, 12.01.2022 14:00







Mathematics, 12.01.2022 14:00

English, 12.01.2022 14:00


Computers and Technology, 12.01.2022 14:00



History, 12.01.2022 14:00

SAT, 12.01.2022 14:00

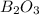 is 60.0 g
is 60.0 g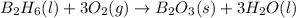
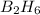 =
=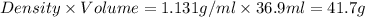
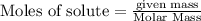
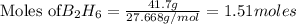
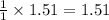 moles of
moles of