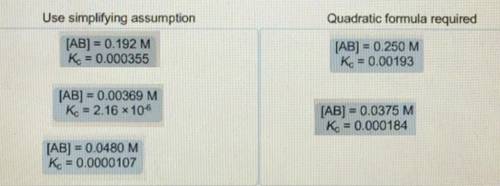
When determining the equilibrium concentrations for the reaction ; a simplifying assumption can be used under certain conditions to avoid solving a quadratic equation. Classify each situation by whether the simplifying assumption can be used or whether the quadratic formula is required. Use simplifying assumptions - Quadratic formula required 1. [AB] = 0.0178 M; 2. [AB] = 0.00204 M; 3. [AB] =0.451 M; = 0.000905 4. [AB] = 0.0174 M; = 0.0000925 5. [AB] = 0.396 M; = 0.00228

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1

Chemistry, 23.06.2019 04:10
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2
You know the right answer?
When determining the equilibrium concentrations for the reaction ; a simplifying assumption can be...
Questions

Arts, 20.12.2020 04:00

Computers and Technology, 20.12.2020 04:00

Physics, 20.12.2020 04:00

History, 20.12.2020 04:00


Chemistry, 20.12.2020 04:00

Physics, 20.12.2020 04:00









Mathematics, 20.12.2020 04:00



Mathematics, 20.12.2020 04:00