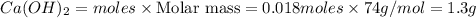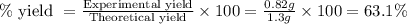Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide:
...

Chemistry, 15.04.2020 03:36 Crtive5515
Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide:
CaO(s)+H2O(l)→Ca(OH)2(s)
In a particular experiment, a 1.00-g sample of CaO is reacted with excess water and 0.82 g of Ca(OH)2 is recovered. What is the percent yield in this experiment?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

You know the right answer?
Questions



Geography, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01


English, 13.10.2020 02:01



Law, 13.10.2020 02:01


Chemistry, 13.10.2020 02:01

English, 13.10.2020 02:01



English, 13.10.2020 02:01



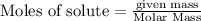
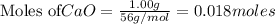
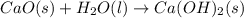
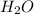 is the excess reagent,
is the excess reagent, 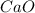 acts as the limiting reagent and it limits the formation of product.
acts as the limiting reagent and it limits the formation of product.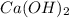
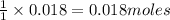 of
of