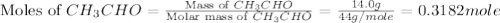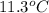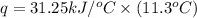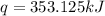Chemistry, 15.04.2020 03:33 tusharchandler124
The heat capacity of a bomb calorimeter was determined by burning 6.91 g of methane (energy of combustion = −803 kJ/mol CH4) in the bomb. The temperature changed by 11.1°C. (a) What is the heat capacity of the bomb? kJ/°C (b) A 14.0-g sample of acetaldehyde (CH3CHO) produced a temperature increase of 11.3°C in the same calorimeter. What is the energy of combustion of acetaldehyde (in kJ/mol)?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Drag each number to the correct location on the equation. each number can be used more than once, but not all numbers will be used. balance the equation with the coefficients. 2 3 4 5 kclo3 -> kcl + o2
Answers: 1


Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

You know the right answer?
The heat capacity of a bomb calorimeter was determined by burning 6.91 g of methane (energy of combu...
Questions










History, 04.06.2020 19:05


English, 04.06.2020 19:05






English, 04.06.2020 19:05


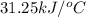
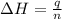
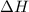 = enthalpy change = -803 kJ/mol
= enthalpy change = -803 kJ/mol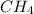 = 6.91 g
= 6.91 g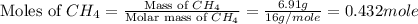
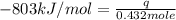
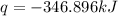
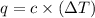
 = change in temperature =
= change in temperature = 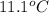
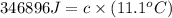
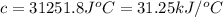
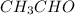 = 14.0 g
= 14.0 g