
If 4.168 kJ of heat is added to a calorimeter containing 75.40 g of water, the temperature of the water and the calorimeter increases from 24.58°C to 35.82°C. Calculate the heat capacity of the calorimeter (in J/°C). The specific heat of water is 4.184 J/g•°C Group of answer choices

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2
You know the right answer?
If 4.168 kJ of heat is added to a calorimeter containing 75.40 g of water, the temperature of the wa...
Questions

Spanish, 02.09.2019 05:30




History, 02.09.2019 05:30


Chemistry, 02.09.2019 05:30

Mathematics, 02.09.2019 05:30



Spanish, 02.09.2019 05:30




History, 02.09.2019 05:30



Mathematics, 02.09.2019 05:30


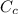 = 54.4
= 54.4 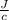
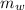 = 75.40 gm
= 75.40 gm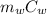 ΔT +
ΔT + 

