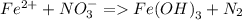Consider the following unbalanced redox reactions. In each case, separate the whole reactions into half-reactions, balance the half reactions, and combine to yield a balanced whole reaction. Compute the net reduction potential (E°) and pε° values for the net reactions and indicate whether the reaction is spontaneous as written. The reduction potential for Fe(OH)_3(s)/Fe^2+ is -0.181 V and pε is -3.08.
a. Fe^2 + + NO_3 = Fe(OH)_3(s) + N_2
b. Mn^2 + + Fe(OH)_3(s) = MnO_2(s) + Fe^2+

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.
Answers: 3

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 23.06.2019 12:30
Is the genie in the bottle experiment a physical or chemical change/reaction?
Answers: 1
You know the right answer?
Consider the following unbalanced redox reactions. In each case, separate the whole reactions into h...
Questions

Mathematics, 12.10.2019 16:50


Mathematics, 12.10.2019 16:50


Advanced Placement (AP), 12.10.2019 16:50

History, 12.10.2019 16:50

English, 12.10.2019 16:50





History, 12.10.2019 16:50


Biology, 12.10.2019 16:50