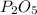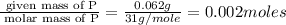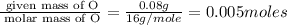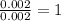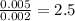Chemistry, 15.04.2020 03:03 frankieflores02
A form of phosphorus called red phosphorus is used in match heads. When 0.062 g of red phosphorus burns in air, it forms 0.142 g of phosphorus oxide. Determine the empirical formula of phosphorus oxide.(show work; use labels)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 06:20
Examine the false statement. compounds are the smallest unit of an element that occur most commonly in nature. select the rewording of the statement that is true. a: atoms are the smallest unit of an element that commonly occur in nature. b: molecules are the smallest unit of an element or compound that commonly occur in nature. c: molecules are the smallest unit of a compound that occur on the periodic table. d: compounds are the smallest unit of an element that occur on the periodic table
Answers: 1
You know the right answer?
A form of phosphorus called red phosphorus is used in match heads. When 0.062 g of red phosphorus bu...
Questions


Mathematics, 01.07.2019 09:30

History, 01.07.2019 09:30


English, 01.07.2019 09:30

Mathematics, 01.07.2019 09:30



History, 01.07.2019 09:30

History, 01.07.2019 09:30

Chemistry, 01.07.2019 09:30


Mathematics, 01.07.2019 09:30