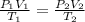Chemistry, 15.04.2020 02:54 laladance123
An ideal gas is contained in a cylinder with a volume of 500 mL at a temperature of 30.0 C and a pressure of 710 torr. The gas is then compressed to a volume of 25 mL, and the temperature is raised to 820 C. What is the new pressure of the gas

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1


Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2

You know the right answer?
An ideal gas is contained in a cylinder with a volume of 500 mL at a temperature of 30.0 C and a pre...
Questions


History, 11.01.2020 07:31

Chemistry, 11.01.2020 07:31

Biology, 11.01.2020 07:31


Mathematics, 11.01.2020 07:31

Mathematics, 11.01.2020 07:31


Mathematics, 11.01.2020 07:31

Mathematics, 11.01.2020 07:31



Mathematics, 11.01.2020 07:31

Medicine, 11.01.2020 07:31


Mathematics, 11.01.2020 07:31




Mathematics, 11.01.2020 07:31