Chemistry, 15.04.2020 03:41 sammysosa121832
The energy needed to ionize an atom of si when it is in the most stable is 786.4 kJ mol^-1 however if an atom of Si is in certain low lying excited state only 310.8 is needed to ionize.
what is the wavelength of he radiation emitted when an atom of si undergoes a transition from this excited state to the ground state?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
When the following equation is balanced using the smallest possible integers, what is the coefficent of oxygen gas? c7h16o(g) + o2(g) → co2(g) + h2o(g) -1 -5 -8 -16 -21
Answers: 3

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1
You know the right answer?
The energy needed to ionize an atom of si when it is in the most stable is 786.4 kJ mol^-1 however i...
Questions


Chemistry, 28.05.2021 20:00

Chemistry, 28.05.2021 20:00



Mathematics, 28.05.2021 20:00

Mathematics, 28.05.2021 20:00

History, 28.05.2021 20:00


Mathematics, 28.05.2021 20:00

Mathematics, 28.05.2021 20:00


Spanish, 28.05.2021 20:00

Computers and Technology, 28.05.2021 20:00

Spanish, 28.05.2021 20:00

Mathematics, 28.05.2021 20:00


English, 28.05.2021 20:00

History, 28.05.2021 20:00

Social Studies, 28.05.2021 20:00

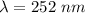
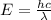
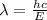 ----- (1)
----- (1)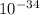 J s
J s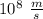
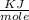
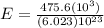
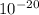 J
J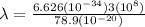
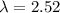 ×
×