
A chemist prepares a solution of magnesium chloride MgCl2 by measuring out 49.mg of MgCl2 into a 100.mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Cl−anions in the chemist's solution. Be sure your answer is rounded to the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:40
Who is better, messi or cristiano, i need this for a chemistry class. asap
Answers: 1


Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1
You know the right answer?
A chemist prepares a solution of magnesium chloride MgCl2 by measuring out 49.mg of MgCl2 into a 100...
Questions




Mathematics, 23.06.2019 02:00



Business, 23.06.2019 02:00

Mathematics, 23.06.2019 02:00

Arts, 23.06.2019 02:00

Mathematics, 23.06.2019 02:00






History, 23.06.2019 02:00



Physics, 23.06.2019 02:00

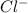 anions in the chemist's solution is 0.0104 M
anions in the chemist's solution is 0.0104 M

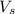 = volume of solution in ml = 100 ml
= volume of solution in ml = 100 ml
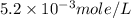

 gives 2 moles of
gives 2 moles of 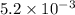 moles of
moles of 


