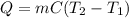In a coffee-cup calorimeter, 100.0 g of H2O and 100.0 mL of HCl are mixed. The HCl had an initial temperature of 44.6 oC and the water was originally at 24.6 oC. After the reaction, the temperature of both substances is 31.3 oC.
a. Was the reaction exothermic or endothermic?Explain.
b. Calculate how much heat the water lost or gained.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2


Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2
You know the right answer?
In a coffee-cup calorimeter, 100.0 g of H2O and 100.0 mL of HCl are mixed. The HCl had an initial te...
Questions

Mathematics, 15.11.2019 03:31


Mathematics, 15.11.2019 03:31

Mathematics, 15.11.2019 03:31



History, 15.11.2019 03:31

Mathematics, 15.11.2019 03:31

History, 15.11.2019 03:31

History, 15.11.2019 03:31




Physics, 15.11.2019 03:31

Mathematics, 15.11.2019 03:31

Physics, 15.11.2019 03:31

Chemistry, 15.11.2019 03:31

Mathematics, 15.11.2019 03:31

History, 15.11.2019 03:31

Biology, 15.11.2019 03:31