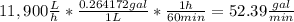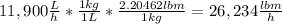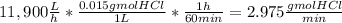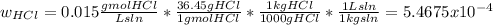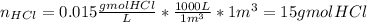The average flowrate of hydrochloric acid solution from a plant is 11,900 Lt/hr. The density is the same as that of water (1kg/Lt). The concentration of HCl in the solution is 0.015gmol/Lt.
Calculate the following:
(i) Flowrate in gallons per minute
(ii) Mass flowrate in lbm / hr
(iii) The number of gram mol of HCl flowing per minute
(iv) The mass fraction of HCl in the solution
(v) The number of lb mols of HCl in 1m3 of solution

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1
You know the right answer?
The average flowrate of hydrochloric acid solution from a plant is 11,900 Lt/hr. The density is the...
Questions


Mathematics, 18.10.2020 03:01


Social Studies, 18.10.2020 03:01


Mathematics, 18.10.2020 03:01



Chemistry, 18.10.2020 03:01




World Languages, 18.10.2020 03:01







Mathematics, 18.10.2020 03:01