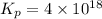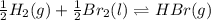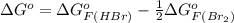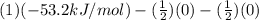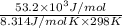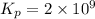Chemistry, 15.04.2020 02:33 karamalqussiri478
Be sure to answer all parts. Be sure to report your answer to the correct number of significant figures. Calculate ΔG o and KP for the following processes at 25°C: (a) H2(g) + Br2(l) ⇌ 2HBr(g) ΔG o = kJ/mol KP = × 10 (Enter your answer in scientific notation.) (b) 1 2 H2(g) + 1 2 Br2(l) ⇌ HBr(g) ΔG o = kJ/mol KP = × 10 (Enter your answer in scientific notation.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2


Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2
You know the right answer?
Be sure to answer all parts. Be sure to report your answer to the correct number of significant figu...
Questions






Mathematics, 01.02.2021 22:10




Mathematics, 01.02.2021 22:10


Biology, 01.02.2021 22:10



Chemistry, 01.02.2021 22:10

Mathematics, 01.02.2021 22:10


Biology, 01.02.2021 22:10


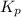 is
is 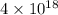 and value of
and value of 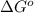 is -106.4 kJ/mol.
is -106.4 kJ/mol.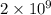 and value of
and value of 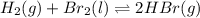
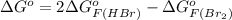
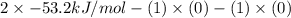
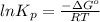
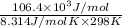
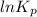 = 42.9
= 42.9