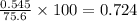Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2
You know the right answer?
Consider the reaction CaCN2 + 3 H2O → CaCO3 + 2 NH3 . This reaction has a 75.6% yield. How many mole...
Questions

Mathematics, 30.10.2019 09:31

Biology, 30.10.2019 09:31











English, 30.10.2019 09:31


Mathematics, 30.10.2019 09:31



Physics, 30.10.2019 09:31

History, 30.10.2019 09:31

World Languages, 30.10.2019 09:31

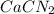 are needed to obtain 18.6 g of
are needed to obtain 18.6 g of 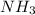
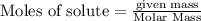
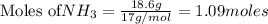
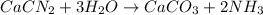
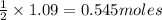 of
of