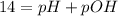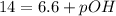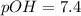Chemistry, 15.04.2020 00:57 sainijasdeep27
In pure water, some of the molecules ionize according to the equation H2O→H+ + OH−. The extent of the ionization increases with temperature. A student heats pure water and records the measured pH at 50°C as 6.6. Based on this information, what mathematical relationships gives the pOH pOH of pure water at 50°C?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

You know the right answer?
In pure water, some of the molecules ionize according to the equation H2O→H+ + OH−. The extent of th...
Questions







Mathematics, 08.10.2021 01:20




English, 08.10.2021 01:20


Computers and Technology, 08.10.2021 01:20




Mathematics, 08.10.2021 01:20

Mathematics, 08.10.2021 01:20

Biology, 08.10.2021 01:20

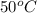 is,
is, 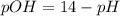
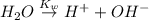
![K_w=[H^+][OH^-]](/tpl/images/0600/4070/bc68a.png)
![\log K_w=\log [H^+]+\log [OH^-]](/tpl/images/0600/4070/f0b12.png)
![-\log K_w=-\log [H^+]+(-\log [OH^-])](/tpl/images/0600/4070/00e23.png)
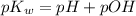
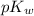 is 14 at 25-50°C.
is 14 at 25-50°C.