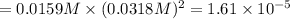PbCl2 (s) ⇆ Pb2+ (aq) + 2Cl− (aq)

Chemistry, 15.04.2020 00:52 genyjoannerubiera
Consider the dissolution equation of lead(II) chloride.
PbCl2 (s) ⇆ Pb2+ (aq) + 2Cl− (aq)
Suppose you add 0.2307 g of PbCl2 (s) to 50.0 mL of water. In the resulting saturated solution, you find that the concentration of Pb2+ (aq) is 0.0159 M and the concentration of Cl− (aq) is 0.0318 M.
What is the value of the equilibrium constant, Ksp, for the dissolution of PbCl2?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3
You know the right answer?
Consider the dissolution equation of lead(II) chloride.
PbCl2 (s) ⇆ Pb2+ (aq) + 2Cl− (aq)
PbCl2 (s) ⇆ Pb2+ (aq) + 2Cl− (aq)
Questions


Mathematics, 01.05.2021 17:50


Mathematics, 01.05.2021 17:50


Computers and Technology, 01.05.2021 17:50








Geography, 01.05.2021 17:50





English, 01.05.2021 17:50

Mathematics, 01.05.2021 17:50

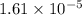 .
.![[Pb^{2+}]=0.0159 M](/tpl/images/0600/3825/89ec5.png)
![[Cl^-]=0.0318 M](/tpl/images/0600/3825/75929.png)
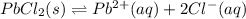
![K_{sp}=[Pb^{2+}][Cl^-]^2](/tpl/images/0600/3825/7fd11.png)