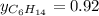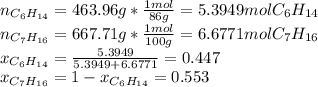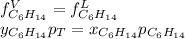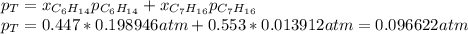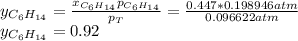Chemistry, 15.04.2020 00:51 malachilaurenc
Two linear hydrocarbons, Hexane (C6H14) and Heptane (C7H16), form pretty much an ideal solution at any composition. A solution is made at 25°C that contains 463.96 g of Hexane in 667.71 g Heptane: Characterise the vapour above this solution, and answer, What is the mole fraction of Hexane in the vapour?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3


Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
Two linear hydrocarbons, Hexane (C6H14) and Heptane (C7H16), form pretty much an ideal solution at a...
Questions

Mathematics, 02.03.2021 18:50

Business, 02.03.2021 18:50


Chemistry, 02.03.2021 18:50

English, 02.03.2021 18:50



Spanish, 02.03.2021 18:50



German, 02.03.2021 18:50

Mathematics, 02.03.2021 18:50

Mathematics, 02.03.2021 18:50





Mathematics, 02.03.2021 18:50

Engineering, 02.03.2021 18:50