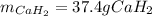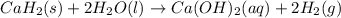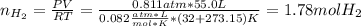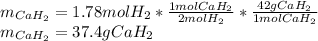Chemistry, 15.04.2020 00:28 swaggirllely36
Calcium hydride (CaH2) reacts with water to form hydrogen gas: CaH2(s) + 2 H2O(l) → Ca(OH)2(aq) + 2 H2(g) Determine the number of grams of CaH2 are needed to generate 55.0 L of H2 gas at a pressure of 0.811 atm and a temperature of 32°C.\

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1


Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
Calcium hydride (CaH2) reacts with water to form hydrogen gas: CaH2(s) + 2 H2O(l) → Ca(OH)2(aq) + 2...
Questions


Mathematics, 07.03.2021 16:50


Mathematics, 07.03.2021 16:50

Chemistry, 07.03.2021 16:50

Chemistry, 07.03.2021 16:50

Arts, 07.03.2021 16:50

English, 07.03.2021 16:50

Mathematics, 07.03.2021 16:50

Advanced Placement (AP), 07.03.2021 16:50

Mathematics, 07.03.2021 16:50


Chemistry, 07.03.2021 16:50


Mathematics, 07.03.2021 16:50

English, 07.03.2021 16:50

World Languages, 07.03.2021 16:50

Advanced Placement (AP), 07.03.2021 16:50