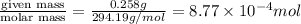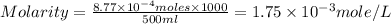Chemistry, 15.04.2020 00:18 sarinaneedshelp01
A chemist prepares a solution by adding 258 mg of K2Cr2O7 (MW = 294.19 g/mol ) to a volumetric flask, and then adding water until the total volume of the contents of the flask reaches the calibration line that indicates 500 mLmL. Determine the molarity of the prepared solution.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2
You know the right answer?
A chemist prepares a solution by adding 258 mg of K2Cr2O7 (MW = 294.19 g/mol ) to a volumetric flask...
Questions



Mathematics, 13.04.2020 23:11




Chemistry, 13.04.2020 23:11


History, 13.04.2020 23:11

Mathematics, 13.04.2020 23:11


Social Studies, 13.04.2020 23:11

Social Studies, 13.04.2020 23:11

World Languages, 13.04.2020 23:11

Chemistry, 13.04.2020 23:11

Mathematics, 13.04.2020 23:12


Mathematics, 13.04.2020 23:12

History, 13.04.2020 23:12

English, 13.04.2020 23:12

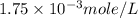
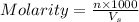
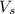 = volume of solution in ml = 500 ml
= volume of solution in ml = 500 ml