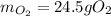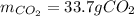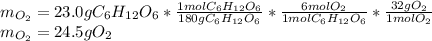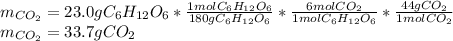Glucose, C6H12O6, is used as an energy source by the human body. The overall reaction in the body is described by the equation:
C6H12O6(aq) + 6O2(g) ⟶ 6CO2(g) + 6H2O(l)
1) Calculate the number of grams of oxygen required to convert 23.0 g of glucose to CO2 and H2O.
2) Calculate the number of grams of CO2 produced.

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3
You know the right answer?
Glucose, C6H12O6, is used as an energy source by the human body. The overall reaction in the body is...
Questions

Geography, 02.03.2021 21:40

Biology, 02.03.2021 21:40


Mathematics, 02.03.2021 21:40

Mathematics, 02.03.2021 21:40

Mathematics, 02.03.2021 21:40

Mathematics, 02.03.2021 21:40

English, 02.03.2021 21:40

Advanced Placement (AP), 02.03.2021 21:40

Biology, 02.03.2021 21:40

Physics, 02.03.2021 21:40

Mathematics, 02.03.2021 21:40


Mathematics, 02.03.2021 21:40

Computers and Technology, 02.03.2021 21:40

Mathematics, 02.03.2021 21:40

History, 02.03.2021 21:40

History, 02.03.2021 21:40

Mathematics, 02.03.2021 21:40