Chemistry, 15.04.2020 02:09 claudia122752
The standard enthalpy change for the following reaction is 232 kJ at 298 K. 2 H2CO(g) 2 C(s, graphite) + 2 H2(g) + O2(g) ΔH° = 232 kJ What is the standard enthalpy change for this reaction at 298 K? C(s, graphite) + H2(g) + 1/2 O2(g) H2CO(g)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:30
If a 60-g object has a volume of 30 cm3, what is its density? 2 g/cm3 0.5 cm3/g 1800 g * cm3 none of the above
Answers: 3

Chemistry, 21.06.2019 14:20
Which is true of chemicals? a. things containing chemicals always cost a lot of money. b. chemicals are never dangerous. c. chemicals are in many substances in a home. d. chemicals are rarely found on earth.
Answers: 1

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

You know the right answer?
The standard enthalpy change for the following reaction is 232 kJ at 298 K. 2 H2CO(g) 2 C(s, graphit...
Questions

Mathematics, 06.12.2021 06:30









Computers and Technology, 06.12.2021 06:40







History, 06.12.2021 06:40



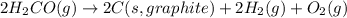
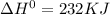
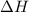 for the following reaction i.e,
for the following reaction i.e,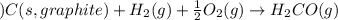
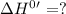
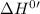 for the reaction will be:
for the reaction will be: