Chemistry, 15.04.2020 02:11 espinosajoselyn
A 5.91 g of octane, C8H18, reacts with excess oxygen in a bomb calorimeter. The heat capacity of the calorimeter is 6.97 kJ/°C and the temperature increases by 32.0°C. How much heat, in units of kJ/mol, was absorbed by the bomb calorimeter?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 05:10
Will mark as brainliest ! how many grams of iron metal do you expect to be produced when 245 grams of an 80.5 percent by mass iron (ii) nitrate solution react with excess aluminum metal? show all work needed to solve this problem!
Answers: 1

Chemistry, 23.06.2019 06:30
(04.01 lc) which of the following is true about science? (5 points) select one: a. it is not influenced by social conditions. b. it is not determined by external local factors. c. political conditions are unable to influence it. d. economic concerns may prevent it from solving problems.
Answers: 1

Chemistry, 23.06.2019 08:00
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2
You know the right answer?
A 5.91 g of octane, C8H18, reacts with excess oxygen in a bomb calorimeter. The heat capacity of the...
Questions


History, 05.05.2020 10:47

History, 05.05.2020 10:47


Social Studies, 05.05.2020 10:47


Biology, 05.05.2020 10:47



History, 05.05.2020 10:47






Mathematics, 05.05.2020 10:47




Spanish, 05.05.2020 10:47

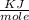
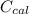 = 6.97
= 6.97 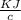
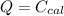 ΔT
ΔT