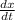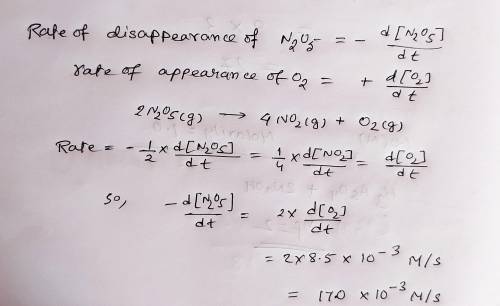Chemistry, 14.04.2020 23:13 jazminemartinez3223
At elevated temperatures, dinitrogen pentoxide decomposes to nitrogen dioxide and oxygen: 2N2O5(g)→4NO2(g)+O2(g) Part A When the rate of formation of O2 is 8.5×10−3 M/s, the rate of decomposition of N2O5 is M/s.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2
You know the right answer?
At elevated temperatures, dinitrogen pentoxide decomposes to nitrogen dioxide and oxygen: 2N2O5(g)→4...
Questions

Mathematics, 11.05.2021 01:00

Mathematics, 11.05.2021 01:00

Mathematics, 11.05.2021 01:00




Health, 11.05.2021 01:00

Mathematics, 11.05.2021 01:00


Mathematics, 11.05.2021 01:00

Mathematics, 11.05.2021 01:00

Mathematics, 11.05.2021 01:00

Mathematics, 11.05.2021 01:00

Mathematics, 11.05.2021 01:00

Mathematics, 11.05.2021 01:00


Mathematics, 11.05.2021 01:00



English, 11.05.2021 01:00